In recent years, the use of exosomes as a targeted drug delivery vehicle has received increasing attention. However, the existing sources of exosomes have several problems, such as low safety and difficulty in large-scale production, and their clinical transformation is difficult. Recently, Dr. Hongzhao Qi at our Institute published a paper in Theranostics, which reported the use of the pH response separation method to obtain blood TfR+ exosomes for tumor-targeted drug delivery. This research contributed to the realization of the clinical transformation of exosomes as nano-drug carriers.

Exosomes (TfR+exosomes) containing transferrin receptors on the surface are abundant in animal blood and have high biological safety. They have good clinical transformation potential as nano-medicine carriers. However, the existing exosomes separation methods have difficulty in achieving the accurate and efficient separation of blood TfR+ exosomes, which has become a bottleneck restricting the study of blood TfR+ exosomes.
In this study, the researchers took full advantage of the pH response characteristics of the combination of transferrin (Tf) and transferrin receptor (TfR) (the mechanism is shown in Figure 1). Under the condition of pH 7.4, saturated transferrin (holo-Tf) could bind TfR. When the pH was adjusted from 7.4 to 5.0, holo-Tf released iron ions to form apo-Tf, but apo- Tf was still bound to TfR. When the pH was reversed from 5.0 to 7.4, apo-Tf was separated from TfR.
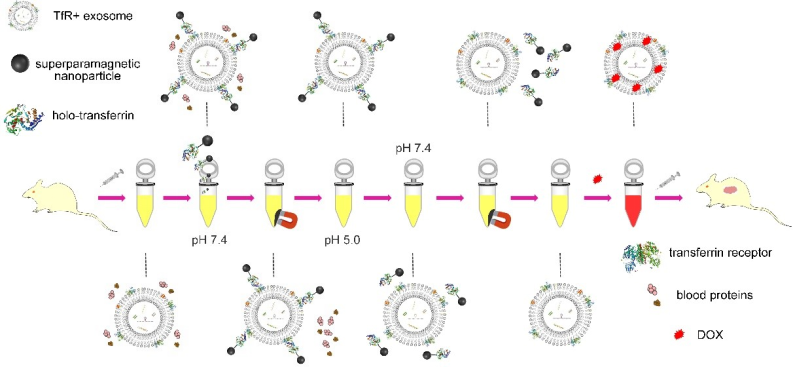
Figure 1. The pH responsiveness of Tf and TfR binding.
Based on this feature, the researchers connected superparamagnetic nanoparticles (SPMNs) with holo-Tf and used the specific binding of Tf and TfR to realize the combination of SPMNs and blood TfR+ exosomes to form a superparamagnetic exosome-based superparamagnetic nanoparticle (SPMN) magnetic nanocluster system. Under the action of an external magnetic field, the system could achieve rapid magnetic separation. At the same time, the pH responsiveness of the combination of Tf and TfR separated SPMNs from the surface of blood TfR+ exosomes, thereby achieving the accurate and efficient separation of blood TfR+ exosomes. The obtained blood TfR+ exosomes efficiently mediated the tumor-targeted therapy of the chemotherapeutic drug Adriamycin. A schematic diagram of the research is shown in Figure 2.
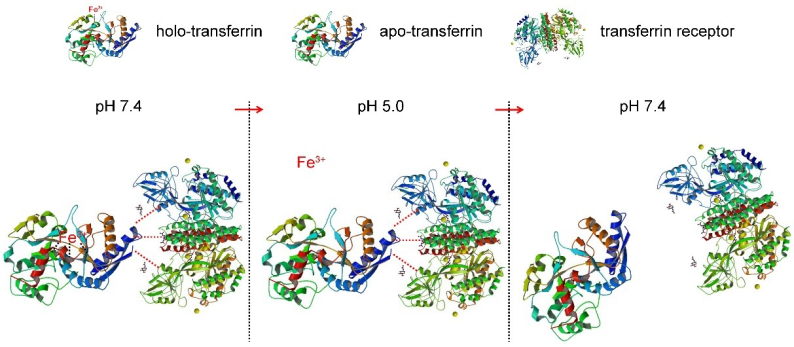
Figure 2. Schematic diagram of the research.
References:
Yang L, Han D, Zhan Q, Li X, Shan P, Hu Y, Ding H, Wang Y, Zhang L, Zhang Y, Xue S, Zhao J, Hou X, Wang Y, Li P, Yuan X, Qi H.Blood TfR+ exosomes separated by a pH-responsive method deliver chemotherapeutics for tumor therapy Theranostics 2019; 9(25):7680-7696. doi:10.7150/thno.37220. Original link: http://www.thno.org/ v09p7680.htm